ACDC-NSYNC

8. Chiral phosphoric acid-catalyzed Friedel-Crafts reaction of 2,5-disubstituted and 2-monosubstituted pyrroles with isoindolinone-derived ketimines
Arben Beriša and Matija Gredičak
Org. Biomol. Chem. 2023, 21, 3381-3387. (PDF)
7. Construction of chiral Betti base precursors containing congested quaternary stereogenic center via chiral phosphoric acid-catalyzed arylation of isoindolinone-derived ketimines
Danijel Glavač and Matija Gredičak
New. J. Chem. 2022, 46, 8760-8764. (PDF)
6. Chemoselective and Regioselective Synthesis of Spiroisoindolinone Indenes
via an Intercepted Meyer-Schuster Rearrangement/Intramolecular Friedel-Crafts Alkylation Relay
Nikola Topolovčan, Marina Degač, Ana Čikoš and Matija Gredičak
J. Org. Chem. 2022, 87, 3712-3717. (PDF)
5. Enantioselective construction of a congested quaternary stereogenic center in
isoindolinones bearing three aryl groups via an organocatalytic formal Betti reaction
Arben Beriša, Danijel Glavač, Chao Zheng, Shu-Li You and Matija Gredičak
Org. Chem. Front. 2022, 9, 428-435. (PDF)
4. Enantioselective construction of a tetrasubstituted stereocenter in isoindolinones via
an organocatalyzed reaction between ketones and 3-hydroxyisoindolinones
Mateja Matišić and Matija Gredičak
Chem. Commun. 2021, 57, 13546-13549. (PDF)
3. Influence of N-Substitution in 3-Alkyl-3-hydroxyisoindolin-1-ones on Stereoselectivity of
Brønsted Acid-Catalyzed Synthesis of 3-Methyleneisoindolin-1-ones
Nikola Topolovčan, Filip Duplić and Matija Gredičak
Eur. J. Org. Chem. 2021, 3920-3924. (PDF)






2. Synthesis and stereoselective catalytic transformations of 3-hydroxyisoindolinones
Nikola Topolovčan and Matija Gredičak
Org. Biomol. Chem. 2021, 19, 4637-4651. (PDF)
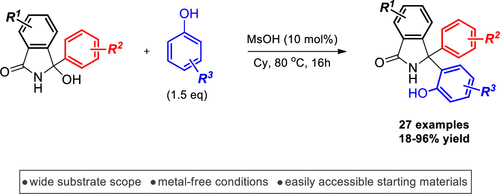
1. Organocatalytic Synthesis of α-Triphenylmethylamines from Diarylketimines and Phenols
Danijel Glavač, Nikola Topolovčan and Matija Gredičak
J. Org. Chem. 2020, 85, 14253-14261. (PDF)